Congenital Protein C Deficiency Treatment Market – Industry Overview, Trends, and Forecast
1. Introduction
Congenital Protein C Deficiency is a rare inherited disorder characterized by the body’s inability to produce sufficient amounts of Protein C, a vital anticoagulant that prevents abnormal blood clotting. The deficiency leads to an increased risk of venous thromboembolism, purpura fulminans, and neonatal thrombosis, particularly in severe cases. The condition is classified into heterozygous and homozygous forms, with the latter being more severe and often presenting symptoms in infancy.
The treatment landscape for Congenital Protein C Deficiency has evolved significantly in recent years, driven by advances in diagnostic tools, protein replacement therapies, and the emergence of recombinant Protein C products. As awareness of rare coagulation disorders increases globally, the Congenital Protein C Deficiency Treatment Market is gaining notable attention from pharmaceutical and biotechnology companies.
This report provides an in-depth analysis of market dynamics, segmentation, regional trends, and the competitive landscape shaping the global industry outlook for the forecast period 2025–2035.
Review comprehensive data and projections in our Global Congenital Protein C Deficiency Treatment Market
report.
Download now: https://www.databridgemarketresearch.com/reports/global-congenital-protein-c-deficiency-treatment-market
2. Market Overview
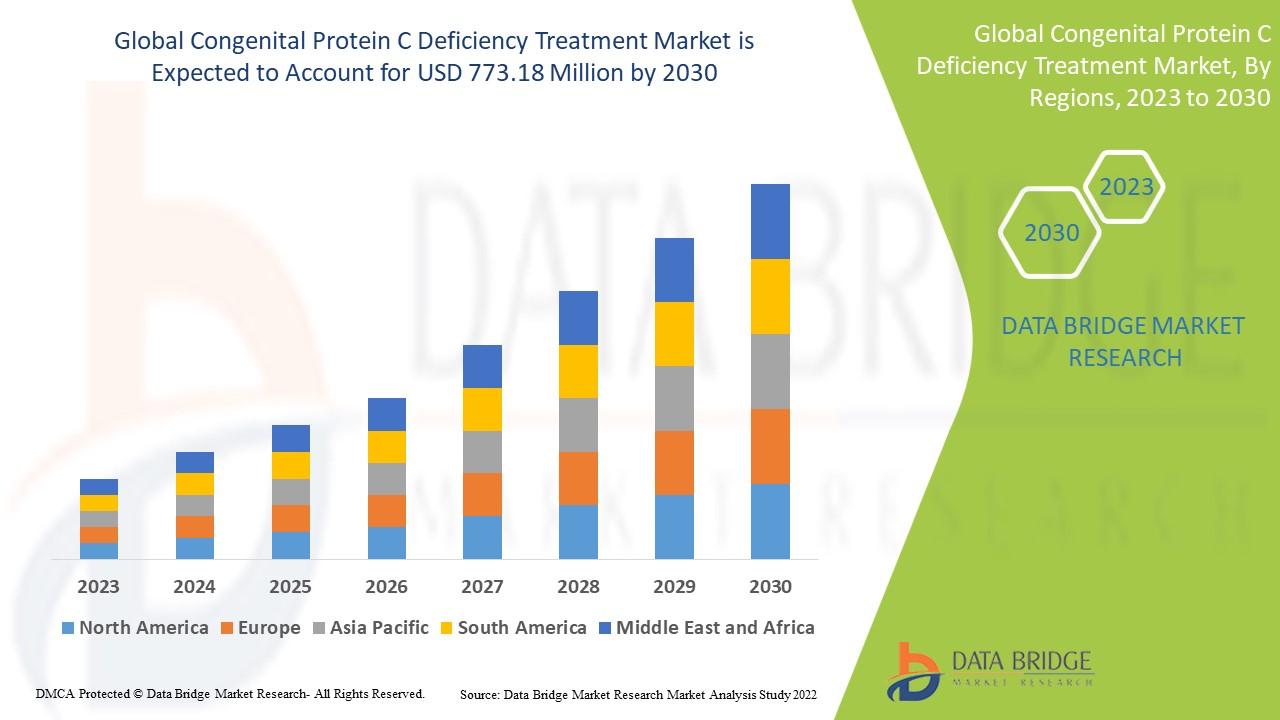
The global Congenital Protein C Deficiency Treatment Market is witnessing steady growth due to a combination of factors such as the rising prevalence of inherited coagulation disorders, increased healthcare spending, and supportive regulatory frameworks for orphan drugs. As of 2024, the market size is estimated at USD XX million, with projections to reach USD XX million by 2035, expanding at a compound annual growth rate (CAGR) of XX% during the forecast period.
Market Drivers
-
Rising awareness and improved diagnosis: Advances in genetic testing and newborn screening have enhanced early detection rates of congenital disorders.
-
Technological innovations: The availability of recombinant Protein C (rProtein C) formulations has improved treatment efficacy and patient safety.
-
Regulatory incentives: Orphan drug designations and government support for rare disease therapies encourage new product development.
Market Challenges
-
High cost of therapy: Treatments involving plasma-derived or recombinant Protein C can be prohibitively expensive for patients in low- and middle-income countries.
-
Limited accessibility: Due to the rarity of the disorder, few specialized treatment centers exist worldwide.
-
Complex manufacturing: Plasma-derived products are highly dependent on donor supply and stringent safety protocols.
Emerging Opportunities
-
Expansion of biopharmaceutical manufacturing in Asia-Pacific.
-
Increasing research in gene therapy and genomic medicine targeting Protein C gene mutations.
-
Growing investments from venture capital firms and orphan drug developers.
3. Market Segmentation
The market is segmented based on treatment type, route of administration, end user, and region.
By Type of Treatment
-
Protein C Concentrate Therapy:
This remains the gold-standard treatment for severe congenital deficiency. Products such as Ceprotin (Takeda) have revolutionized management by offering precise dosing and rapid action. -
Anticoagulant Therapy:
Includes heparin and warfarin, used primarily in heterozygous cases or as adjunct therapy. -
Fresh Frozen Plasma (FFP):
Used as an emergency substitute when specific concentrates are unavailable, especially in low-resource settings. -
Other Supportive Treatments:
Includes vitamin K supplementation and novel anticoagulant drugs under clinical trials.
By Route of Administration
-
Intravenous: Dominant segment, primarily for Protein C concentrates and plasma transfusions.
-
Oral: Growing adoption in anticoagulant management due to patient convenience.
-
Others: Emerging administration methods under research, including subcutaneous and sustained-release formulations.
By End User
-
Hospitals: Largest market share due to availability of specialized care and plasma-based therapies.
-
Specialty Clinics: Focused centers for rare disease management and long-term monitoring.
-
Homecare Settings: Gaining traction for mild cases through anticoagulant therapy and telemedicine support.
-
Research Institutions: Key contributors to ongoing clinical and translational research.
4. Competitive Landscape
The Congenital Protein C Deficiency Treatment Market features a small but competitive set of players focusing on rare disease innovation and advanced therapeutic development.
Key Market Players:
-
Takeda Pharmaceutical Company Limited
-
CSL Behring
-
Grifols S.A.
-
Octapharma AG
-
Baxter International Inc.
-
Kedrion Biopharma
-
Pfizer Inc.
-
Novo Nordisk A/S
Recent Developments
-
Takeda’s Ceprotin remains one of the leading FDA-approved Protein C concentrates.
-
CSL Behring is expanding its recombinant product pipeline to improve sustainability and reduce reliance on plasma-derived therapies.
-
Octapharma is investing in plasma collection infrastructure to meet growing global demand.
-
Biotech start-ups are exploring gene-editing platforms (e.g., CRISPR/Cas9) for potential curative therapies.
Strategic Initiatives
-
Partnerships with academic institutions for clinical trials.
-
Government collaborations under orphan drug programs.
-
Mergers and acquisitions to strengthen product portfolios and expand geographic presence.
5. Market Dynamics
Key Drivers
-
Increasing diagnosis rates owing to the availability of molecular diagnostic technologies.
-
Advancements in recombinant therapy enhancing safety and consistency over plasma-derived products.
-
Policy support from regulatory agencies such as the FDA and EMA promoting orphan drug approvals.
Restraints
-
High manufacturing and therapy costs, restricting access for developing countries.
-
Limited patient population, which impacts return on investment for manufacturers.
-
Supply chain vulnerabilities in plasma product sourcing and distribution.
Opportunities
-
Gene therapy breakthroughs providing potential curative options.
-
Emerging markets in Asia-Pacific and Latin America offering untapped growth potential.
-
Public-private collaborations accelerating clinical research and awareness programs.
Trends
-
Increasing adoption of personalized medicine approaches.
-
Integration of digital health tools for patient monitoring and data collection.
-
Focus on sustainable manufacturing of recombinant proteins.
6. Regional Insights
North America
North America holds the largest market share, driven by strong research infrastructure, favorable reimbursement frameworks, and active clinical trials. The U.S. remains a major hub for orphan drug development, with the FDA providing accelerated approval pathways for rare disease therapies.
Europe
Europe follows closely, supported by robust healthcare systems and EU-funded rare disease initiatives. Countries such as Germany, the U.K., and France are investing heavily in advanced protein replacement and plasma collection systems.
Asia-Pacific
The Asia-Pacific region is expected to witness the fastest growth over the forecast period. Rising healthcare investments, expanding patient registries, and growing awareness in countries like India, China, and Japan are fueling demand for specialized treatment options.
Latin America
The market is gradually expanding in Latin America as healthcare infrastructure improves and government programs for rare disease management gain traction. However, cost and access remain significant challenges.
Middle East & Africa
This region remains underpenetrated but presents future growth potential with ongoing public health initiatives and international collaborations aimed at improving rare disease care.
7. Future Outlook
The future of the global Congenital Protein C Deficiency Treatment Market appears promising, with innovation at the forefront. The increasing focus on biopharmaceutical research, precision medicine, and genetic therapies is expected to redefine treatment paradigms.
By 2035, the market is anticipated to achieve robust growth as recombinant and gene-based therapies transition from clinical trials to commercialization. Additionally, policy incentives such as tax credits and fast-track approvals will continue to attract investment in rare disease therapeutics.
The integration of digital health solutions — including AI-powered diagnostic platforms and real-world data monitoring — will enhance patient outcomes and accelerate clinical decision-making. Ultimately, the combination of technological advancement and global collaboration is set to drive the next phase of market evolution.
8. Key Takeaways
-
The market is expanding steadily due to advances in recombinant Protein C therapies and rare disease awareness.
-
North America currently leads the global market, followed by Europe.
-
Gene therapy and personalized medicine represent the next frontier in treatment innovation.
-
High therapy costs and limited access continue to challenge market penetration.
-
Strategic partnerships and R&D investments are key to unlocking long-term growth potential.
9. Frequently Asked Questions (FAQs)
1. What is Congenital Protein C Deficiency?
It is a rare genetic disorder caused by a deficiency of Protein C, leading to excessive blood clot formation and associated complications such as purpura fulminans and thrombosis.
2. What are the main treatment options?
Treatment typically involves Protein C concentrate infusions, anticoagulant therapy (heparin or warfarin), and, in some cases, fresh frozen plasma.
3. Which factors are driving market growth?
Increased awareness of rare diseases, improved diagnostic capabilities, and advances in recombinant therapy are major growth drivers.
4. Who are the leading companies in the market?
Key players include Takeda, CSL Behring, Octapharma, Grifols, and Baxter International.
5. Which region dominates the global market?
North America leads due to advanced healthcare systems and supportive regulatory environments.
6. What are the major challenges?
High treatment costs, limited availability of specialized care, and complex plasma collection processes are key challenges.
7. What is the future outlook for the market?
The market is expected to grow steadily, with emerging trends such as gene therapy, digital health integration, and expansion in developing regions shaping its evolution.
Browse More Reports:
Europe A2 Milk Market
Asia-Pacific A2 Milk Market
North America and Europe Celiac Disease Market
North America Methotrexate Injection Market
North America Water Dispensers Market
Global Active Medical Implantable Devices Market
Global Adult Malignant Glioma Therapeutics Market
Global Air Brake System Market
Global Air Fresheners Market
Global Airsoft Guns Market
Global Alkylation Market
Global Anticoagulation Therapy Market
Global Archery Equipment Market
Global Arthritis Market
Global Asset Tracking and Inventory Management Solutions Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com